491
Views & Citations10
Likes & Shares
INTRODUCTION
Brucellosis almost always causes fever, which may be associated with night sweats and weight loss [3-6]. Common treatment includes doxycycline and aminoglycosides, although complicated cases, such as brucellosis combined with endocarditis, neurobrucellosis, may require stronger medicatinos administered for a longer duration and challenging treatment process [7-9]. Other than direct contact with infected animals, the disease is also foodborne; food habits are very difficult to change, in eastern of Turkey also cheese made with unboiled milk, which will assure many future cases of foodborne disease [10,11]. Brucella melitensis, Brucella abortus and Brucella suis are the three species generally associated with human disease, in our region B. melitensis common cause of the brucellosis. Rare cases of human infection with Brucella canis have been reported, while human cases of Brucella ovis and Brucella neotomae infection have not been reported. Little is known about the capacity of the new Brucella species to cause infection [10]. One possible laboratory acquired infection with a marine mammal isolate has been reported [12] and one specific sequence type (ST27) has been associated with three human infections in Peru and New Zealand [10,12]. Interestingly, the patients had had no contact with marine mammals; however contact with raw fish was a common feature of the three cases of patients. B. melitensis bv2 found in catfish in Egypt, suggesting that fish may constitute a novel source of infection [10,13]. There is some other types of brucella; Brucella canis, Brucella suis, Brucella ovis, Brucella ceti, Brucella pinnipedialis, Brucella cetaceae, Brucella neotomae [14]. In endemic areas like our region Brucella melitensis is the most common type [15].
The aim of this study was to investigate the seroprevalence of brucellosis titters in 5 years patients with using the rose Bengal agglutination test and coombs brucella test. Coombs brucella test is a microaglutination test as sandwich ELISA model. The sensitivity and specifity are found to be 94-95% and 98-99%. The aglutinin detect IgG, IgM and IgA. Test is effective in detecting the agglutinating and non-agglutinating immunglobulins in acute and chronic brucellosis caused by B. abortus, B. melitensis and B. suis.
MATERIALS AND METHOD
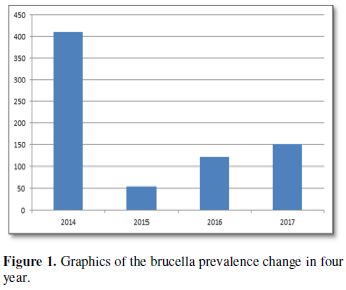
A total of 761 brucellosis cases admitted to our clinic, the Department of Infectious Diseases and Clinical Microbiology of Kafkas University Hospital, over a 4 year period from 2014 to 2017, were included in the study. A retrospective study was undertaken and patient files were investigated for their gender, age, rose Bengal positivity and brucella coombs titters, as well as clinical outcomes. Firstly rose Bengal plate test, the procedure described by Foster et al. [15] was followed with little modification by Unver et al. [16]. Brucella Rose Bengal Plate Test antigen was prepared from Brucella abortus S99 strain, standardized with Brucella antiserum and stained with Rose-Bengal. The antigen was left at room temperature for 15 min before use and shaken well. On a clean plate, 0.05 ml milk serum was dropped. Then, 0.05 ml Rose Bengal Lam Test antigen was added to this. The antigen and milk serum were mixed and spread over an area with diameter 1.5 cm. The plate was left in the air for 2 min by turning by hand. Formation of clusters of coarse particles was assessed as positive while homogeneous
After the agglutination Brucella coombs test were performed. The procedure of test: two wells are used for each serum and well for positive control and well 1 for negative control are used for each run.
· 5 μl of serum is diluted with 195 μl saline solution in a test tube (1:40 titer). Mix well while diluting the serum sample.
· 2 wells for each serum sample and 2 wells for positive-negative control are emplaced to the microplate frame and 50 μl MCBT diluent is added to the each well.
· The diluted serum sample is added to first well, which 50 μl of (1:80 titer). It is mixed well by pipetting 3-4 times than 50 μl of 1:80 diluted sample is added to the other well (1:160 titer), 50 μl of diluted sample is discarded from last well.
· 5 μl of positive and negative control solution is added to the control wells.
· 50 μl of antigen is added to all wells (First well 1:160 titer, second well 1:320 titer).
· The plate is incubated in the special locked box with a piece of wet cotton for 24 h.
· The results are evaluated after the incubation period. Solutions are in order: 1/40, 1/80, 1/320, 1/640, 1/1280, 1/2560, 1/5120.
RESULTS
DISCUSSION AND CONCLUSION
Brucellosis is the most frequent zoonotic infectious disease in the world, affecting more than 500 000 people every year [16]. In the eastern part of Turkey, seropositivity has been reported to be as high as 27.2% [17]. In endemic countries, brucellosis is more prevalent age group is 15-35 [18,19]. In our study most of cases are between 18-35 years old age too but more than 35 years old patient’s rates are high to undeniable. In our geographic region Kafkas basin the most common route of spread is the consumption of regional milk products produced from raw milk like Van lake basin [20]. In a study from Spain which is epidemic region for brucellosis, the sensitivity value for SAT was found as 65.8%, for Coombs test as 91.5% and for BrucellaCapt as 95.1% [16]. In another study from same region the sensitivity and specificity values for Rose Bengal, SAT, Coombs Test and Brucellacapt was found over 90% whereas IgM and IgG ELISAs have the lowest sensitivity (60% and 84%, respectively), gold standart is culture of bacteria of course [21,22]. In our study Brucella Rose Bengal test is used first and then BrucellaCapt used for titers 761 patient found positive in four year period. Most of the patients coming from our region because of the location of our city. In the similar study in Erzurum they found a significant difference between the two regions among the Hinis and Oltu individuals aged 10-20 and 20-60 provinces which people use boiling dairy products and no boiling products [11].
The high individual prevalence of brucella seropositivity in people in the intensive dairy farm owners around Kars agree with the characteristics of these husbandry systems, the levels of brucella infections tend to be relatively high on intensive farms, whether these have indigenous cattle or introduced sheep. The first stage in controlling zoonotic diseases is periodic observation of the prevalence and distribution of the diseases in animals, making timely intervention against epidemics, vaccination, and prevention of the disease in animals possible for these zoonotic diseases.
In multiple analyses, the relations disappeared regarding to close contact with animals and occupation while the other relations remained. Data of 761 patients were completed and analyzed in the study. Of them, 77% were males and 22% were females. Most of them are farmers and animal owners.
1. Sandra C, Savic S, Cauchi D,
Lautier E, Canali M, et al. (2018) Brucellosis control in Malta and Serbia: A
one health evaluation frontiers in veterinary science. Front Vet Sci 5: 147.
2. Sarı E, Özgen İ, Say A,
Güven F, Ulutaş P (2013) The evaluation of brucellosis in children in an
endemic region of Turkey, Van. Gaziantep Med J 19: 1-4.
3. Lim HS, Song YG, Yoo HS,
Park MY, Kim JW (2005) Brucellosis: An overview. Korean J Epidemiol 27: 26-36.
4. Guerra H (2007) The
brucellae and their success as pathogens. Crit Rev Microbiol 33: 325-331.
5. Jang Y, Kim H, Bang HA, Lee
MJ, Che NH, et al. (2011) Epidemiological aspects of human brucellosis and
leptospirosis outbreaks in Korea. J Clin Med Res 3: 199-202.
6. Kim SH, Kim KP, Han S, Kim
YR, Kang SH (2015) A case of acute myeloid leukemia developing after treatment
for brucellosis with pancytopenia. Lab Med Online 5: 157-160.
7. Ariza J, Bosilkovski M,
Cascio A, Colmenero JD, Corbel MJ, et al. (2007) Perspectives for the treatment
of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med 4:
e317.
8. Ablon G, Dayan S (2015) A
randomized, double-blind, placebo-controlled, multi-center, extension trial
evaluating the efficacy of a new oral supplement in women with self-perceived
thinning hair. J Clin Aesthet Dermatol 8: 15-21.
9. del Pozo JSG, Solera J
(2012) Systematic review and meta-analysis of randomized clinical trials in the
treatment of human brucellosis. PLoS One 7: e32090.
10. Ahn AN, Ann HW, Jung IY, Jung
W, Lee JY, et al. (2018) Imported case of Brucella
melitensis ınfection in South Korea. Infect Chemother 50: 149-152.
11. Yumuk Z, O’Calaghan D (2012)
Brucellosis in Turkey. Int J Infect Dis 16: e228-e235.
12. Celebi Ö, Celebi D, Balkan CE
(2013) Effects of boiling dairy products on human brucellosis. Eurasian J Med
45: 73-76.
13. Whatmore AM, Dawson CE,
Groussaud P, Koylass MS, King AC, et al. (2008) Marine mammal Brucella genotype
associated with zoonotic infection. Emerg Infect Dis 14: 517-518.
14. El-Tras WF, Tayel AA,
Eltholth MM, Guitian J (2010) Brucella infection in fresh water fish: Evidence
for natural infection of Nile catfish, Clarias
gariepinus, with Brucella melitensis.
Vet Microbiol 141: 321-325.
15. Foster G, Osterman BS,
Godfroid J, Jacques I, Cloeckaert A (2007) Brucella
ceti sp. nov. and Brucella
pinnipedialis sp. nov. for Brucella strains with cetaceans and seals as
their preferred hosts. Int J Syst Evol Microbiol 57: 2688-2693.
16. Unver A, Erdogan HM, Atabay
HI, Sahın M, Celebl O (2006) Isolation, identification and molecular
characterization of Brucella melitensis
from aborted sheep fetuses in Kars, Turkey. Revue Méd Vét 157: 42-46.
17. Pappas G, Papadimitriou P,
Akritidis N, Christou L, Tsianos EV (2006) The new global map of human
brucellosis. Lancet Infect Dis 6: 91-99.
18. Ceylan E, Irmak H, Buzgan T,
Karahocagil MK, Evirgen O, et al. (2003) Van iline bag˘lı bazı köylerde insan
ve hayvan populasyonunda bruselloz seroprevalansı. Van Tıp Derg 10: 1-5.
19. Young EJ (2005) Brucella
species. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice
of infectious diseases. 6th Edn. Philadelphia: Churchill
Livingstone, pp: 2669-2672.
20. Doğanay M, Alp E (2008)
Infeksiyon hastalıkları ve mikrobiyolojisi. In: Topcu AW, Söyletir G, Doğanay
M, editors. 3rd Edn. Istanbul: Nobel Tıp Kitabevleri, pp: 897-909.
21. Gomez MC, Nieto JA, Rosa C,
Geijo P, Escribano MA, et al. (2008) Evaluation of seven tests for diagnosis of
human brucellosis in an area where the disease is endemic. Clin Vacc Immunol
15: 1031-1033.
22. Omer MK, Skjerve E, Holstad
G, Woldehıwet Z, Macmıllan AP (2000) Prevalence of antibodies to Brucella spp. in cattle, sheep, goats,
horses and camels in the State of Eritrea; influence of husbandry systems.
Epidemiol Infect 125: 447-453.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Astronomy and Space Research
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Proteomics and Bioinformatics (ISSN:2641-7561)